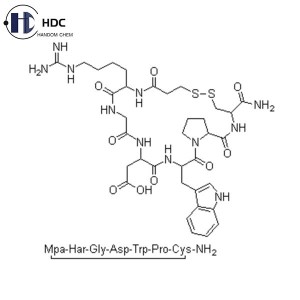
Eptifibatide Acetate
Brief Introduction:
Eptifibatide is a synthetic cyclic heptapeptide, a glycoprotein IIb/IIIa receptor antagonist and a fibrinogen receptor antagonist with anti-platelet aggregation effects. Its main function is anti-platelet aggregation and can be used for anti-thrombotic treatment in acute coronary syndrome.
Specifications of our Eptifibatide Acetate:
| Test Items | Specifications |
| Characteristic | This product is a white or off-white amorphous powder; odourless and hygroscopic. It is easily soluble in water and almost insoluble in chloroform or acetone. |
| Specific Rotation | Calculated based on anhydrous and acetic acid-free product, the specific rotation is between -52.0° and -56.0° |
| Identification | (1) This product has maximum absorption at wavelengths of 220nm and 280nm |
| (2) The retention time of the main peak of the test solution should be consistent with the retention time of the main peak of the reference solution | |
| (3) The infrared absorption spectrum of this product should be consistent with the spectrum of eptifibatide reference substance | |
| (4) The mass spectrum molecular weight should be 832±1Da | |
| Solution Clarity and Colour | The solution should be clear and colourless. If it is turbid, it should not be darker than the No. 2 turbidity standard solution; if it is coloured, it should not be darker than the yellow No. 1 or yellow-green No. 1 standard colorimetric solution. |
| Acidity | The pH value should be between 3.5 and 5.5 |
| Moisture | Not more than 5.0% |
| Acetic Acid | Not more than 12.0% |
| Trifluoroacetic Acid | Not more than 0.5% |
| Residual Solvents | Methanol: Not more than 0.3% |
| Acetonitrile: Not more than 0.041% | |
| N,N-Dimethylformamide: Not more than 0.088% | |
| Amino Acids Ratio | Asparatic Acid (Asp): 0.8 ~ 1.2 |
| Homoarginine (H-HomoArg-OH): 0.8 ~ 1.2 | |
| Glycine (Gly): 0.8 ~ 1.2 | |
| Proline (Pro): 0.8 ~ 1.2 | |
| Tryptophan (Trp): 0.8 ~ 1.2 | |
| Cysteine (Cys): 0.8 ~ 1.2 | |
| Related Substances | The peak area of impurity A and impurity H shall not be greater than 1.5 times (0.3%) of the main peak area of the control solution. The peak area of impurity B, impurity C, impurity D and impurity E multiplied by the correction factor (correction factor 1.23), impurity F (correction factor 0.86) and impurity G (correction factor 1.24) shall not be greater than the main peak area of the control solution (0.2%); The peak area of other single unknown impurities shall not be greater than the main peak area of the control solution (0.2%), and the sum of the peak areas of each impurity shall not be greater than 5 times the main peak area of the control solution (1.0%) |
| Polymer | The retention time of each component less than that of eptifibatide impurity G must not exceed 0.5%, and the total impurities should not exceed 1.0% (including impurity G) |
| Arsenic salt | Not more than 0.00015% |
| Bacterial endotoxins | The amount of endotoxin contained in every 1 mg of eptifibatide should be less than 0.2 EU |
| Microbial Limits | Total Aerobic Microbial Count: NMT 1000 CFU/g |
| Total Yeasts and Moulds Count: NMT 100 CFU/g | |
| Bile-tolerant Gram-negative Bacteria | |
| Assay | Calculated as anhydrous and acetic acid-free, this product contains eptifibatide 98.0% ~ 102.0% |
Indications:
This product is suitable for patients with coronary syndrome, regardless of whether they have acute coronary symptoms (unstable angina and myocardial infarction without Q wave), as well as those patients who have acute coronary symptoms and are taking drug treatment.
Packaging:
1g/bottle, 5g/bottle, 10g/bottle, 30g/bottle, 50g/bottle or 100g/bottle.
Storage Conditions:
Stored in unopened original containers in a cool dry place before using; kept away from direct sunlight, heat and moisture; preserved at 2℃ to 8℃ for short-term storage, -20℃±5℃ for long-term storage.
Shelf Life:
24 months if stored under above mentioned conditions.